Schedule a Call Back
PDG welcomes Indian Pharmacopoeia Commission as a member
 Industry News
Industry News- Oct 13,23

The Pharmacopoeial Discussion Group (PDG) announced the Indian Pharmacopoeia Commission (IPC) as a PDG member on October 5, 2023 during PDG Stakeholder?s meeting in Hyderabad. IPC officially joined as a member in the PDG at the PDG?s Annual Meeting which was held on October 3-4, 2023 in Hyderabad. The World Health Organisation (WHO) also continues to serve as observer of the PDG.
Pharmacopoeial Discussion Group (PDG) The PDG, will now bring together the European Pharmacopoeia (Ph. Eur.), the Japanese Pharmacopoeia (JP), the United States Pharmacopeia (USP) and Indian Pharmacopoeia (IP) to harmonise global pharmacopoeial standards. The objective is to reduce manufacturers' burden of having to perform analytical procedures in different ways, using different acceptance criteria, in order to satisfy pharmacopoeial requirements that vary across regions. PDG has defined harmonisation of a pharmacopoeial monograph or general chapter as follows: ?A pharmacopoeial general chapter or other pharmacopoeial document is harmonised when a pharmaceutical substance or product tested by the document's harmonised procedure yields the same results and the same accept/reject decision is reached.? IPC?s Participation in PDG Pilot for Global Expansion IPC was only Pharmacopoeia body in the world to be selected for pilot phase initiated in September 2022. After reviewing each application, the PDG agreed by consensus, to start the pilot phase with the IPC, the only applicant that met all the requirements in the entry criteria for the pilot. This represented IPC?s continued commitment and capability to develop world class quality standards for drugs and pharmaceuticals. Declaration of IPC as Member by the PDG After 1 year of pilot phase, based on IPC?s involvement, contribution and future potential, the decision on inclusion as permanent member of PDG was taken. An official letter was sent to IPC by the PDG on September 18, 2023 regarding confirmation of membership of IPC in the PDG. Global Impact of IPC as PDG Member i. Inclusion of IP in PDG will significantly increase the visibility of the Indian Pharmacopoia on international platform. It will establish IP as progressive pharmacopoeia which designs drug quality standards at par with global standards. Application of these standards will lead to production of world class pharmaceutical products for domestic and export markets. ii. IPC?s inclusion in PDG will help in effort towards its recognition by other countries. iii. Harmonisation of Standards: It will help IPC to collaborate and harmonise pharmacopoeial standards with other major regulatory/standard setting authorities which in turn will help in ensuring the quality and safety of pharmaceuticals at a global level. iv. International Recognition: Membership in the PDG would enhance the international recognition of the standards set by the IPC. This also has potential to improve the acceptance of Indian pharmaceutical products in global markets, as they adhere to internationally recognised quality standards. v. Improved Regulatory Compliance: IPC will benefit from the exchange of information and best practices with other PDG members. This collaboration will help India in aligning its regulatory processes and practices with global standards, making it easier for Indian pharmaceutical companies to comply with international regulations. vi. Access to Global Markets: Membership in the PDG will facilitate enhanced export of Indian pharmaceutical products to other member countries. Aligning with international standards will reduce trade barriers and make it easier for Indian pharmaceutical companies to access global markets. vii. Global Health Impact: The harmonisation of pharmacopoeial standards among PDG members will contribute to the global effort to ensure the safety and efficacy of marketed pharmaceutical products. This will have a direct impact on public health worldwide, as it helps to prevent the circulation of substandard or counterfeit drugs. IPC?s membership in the PDG is one step forward towards promoting harmonisation of pharmaceutical standards, improving regulatory compliance, facilitating international recognition, and ultimately contributing to global public health through the assurance of drug quality and safety. Source: PIB
Related Stories
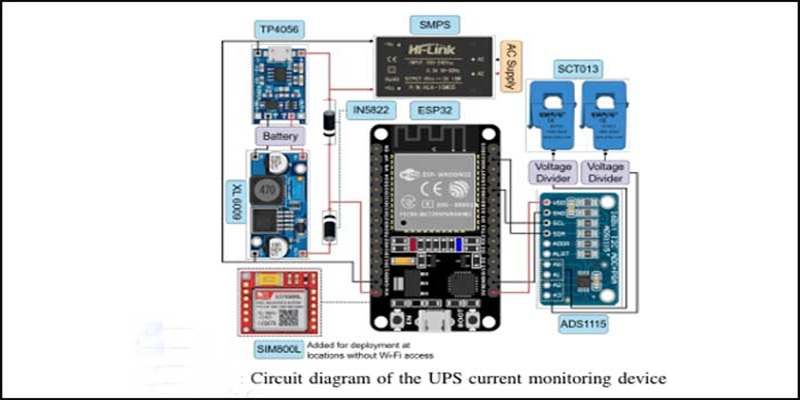
Low-Cost IoT Device Enables Real-Time UPS Monitoring at IIIT-H
Born from a campus IT challenge, IIIT-H researchers have developed Rs 2,000 IoT device that monitors UPS performance during power outages with near-second accuracy.
Read more
Raghu Vamsi launches DeepTech plant, Unveils UAVs India Asia
Raghu Vamsi Aerospace Group announced a new DeepTech facility near Hyderabad and unveiled six India-made UAV and autonomous defence systems, backed by over Rs 1 billion investment, strengthening sel..
Read more
Raghu Vamsi launches DeepTech plant, Unveils UAVs India Asia
Raghu Vamsi Aerospace Group announced a new DeepTech facility near Hyderabad and unveiled six India-made UAV and autonomous defence systems, backed by over Rs 1 billion investment, strengthening sel..
Read moreRelated Products

Programmable Controllers - Pcd-33a Series
Pro-Med Instruments (P) Ltd offers a wide range of programmable controllers - PCD-33A Series.

Gasket Graphite Powder
Arihant Packing & Gasket Company offers a wide range of gasket graphite powder.
Asahi Kasei expands 3D printing filament sales in North America
Asahi Kasei, a leading resin and compounding technology provider, has initiated the sales of 3D printing (3DP) filaments in North America through Asahi Kasei Plastics North America (APNA). The soft la Read more
















