Schedule a Call Back
Yokogawa unveils OpreX to accelerate DX in quality assurance
 Industry News
Industry News- Feb 22,25
Related Stories

REC Facilitates MoU for NABL-Accredited Testing Facility in Meghalaya’s Power Sector
REC Limited has mediated a key MoU between MePDCL and CPRI to establish a NABL-accredited testing facility in Meghalaya, boosting local power infrastructure quality and accelerating RDSS projects.
Read more
Renishaw Unveils Equator–X 500 Dual-Method Gauging System
Equator–X 500 dual-method system extends the Equator range of versatile gauges for shop floor process control, high-speed measurement and quality assurance
Read more
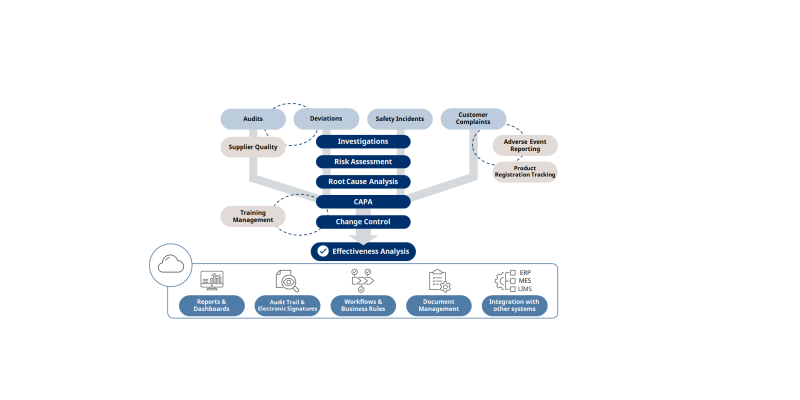
Yokogawa unveils OpreX to accelerate DX in quality assurance
The audit trail and user management methods employed by this system comply with global pharmaceutical regulations, leading to improved integrity in the handling of data for quality assurance proce..
Read moreRelated Products

Programmable Controllers - Pcd-33a Series
Pro-Med Instruments (P) Ltd offers a wide range of programmable controllers - PCD-33A Series.

Gasket Graphite Powder
Arihant Packing & Gasket Company offers a wide range of gasket graphite powder.
Asahi Kasei expands 3D printing filament sales in North America
Asahi Kasei, a leading resin and compounding technology provider, has initiated the sales of 3D printing (3DP) filaments in North America through Asahi Kasei Plastics North America (APNA). The soft la Read more














