Schedule a Call Back
Indian medical device makers seek extension for Class C, D device licensing
 Industry News
Industry News- Oct 06,23

Medical device manufacturers have formally requested a six-month extension to comply with mandatory licensing for Class C and D medical devices, as mandated by the Drugs and Cosmetics Act. The Association of Indian Medical Device Industry (AiMED), representing domestic medical device makers, sent a letter dated September 25 to Health Minister Mansukh Mandaviya, outlining their concerns. They cited resource constraints within the Central Drugs Standard Control Organisation (CDSCO), hindering timely inspections and issuance of manufacturing licenses before the October 1 deadline.
In the letter, Rajiv Nath, the forum coordinator of AiMED, explained the need for an extension, expressing worries that CDSCO's limited resources might lead to supply chain disruptions for numerous Indian-made medical devices. According to the New Medical Devices Regulations, 2020, manufacturers were given a 42-month transition period, starting from April 1, 2020, to complete licensing for non-notified Class C and D medical devices.
An industry expert highlighted the need for clarity regarding high-risk medical devices. He estimated that around 1,000 manufacturing licenses are in process for 200-300 manufacturers producing these high-end medical devices. Starting from October 1, all Class C and D devices are to be subject to licensing requirements under non-regulatory medical devices.
Without proper licenses, legal sales cannot proceed, and manufacturers must halt billing until they obtain a manufacturing license. Manufacturers have sought clarification from CDSCO and the Union health ministry regarding whether registered manufacturers can conduct sales in the absence of a license. The medical devices falling under Class C and D categories include critical equipment such as defibrillators, ventilators, imaging devices, oxygen therapy equipment, and nebulizers.
Source: Business Standard
Related Stories
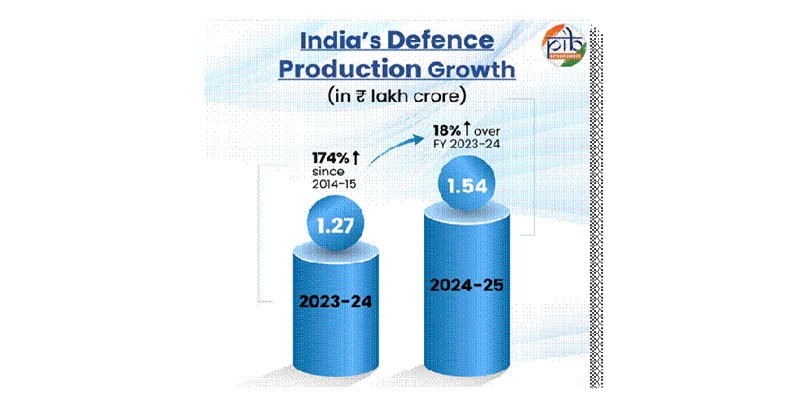
India’s Defence Production Hits Rs 1.27 Trillion, Driven by Reforms
Record output reflects decade of policy reforms, rising exports and strong domestic industry.
Read more
Imports of medical device surged in India in fy23-34 to Rs 688.85 billion
This marks a 13% increase, with disposables accounting for nearly 17.6% of the growth.
Read more
Female Apprenticeship surges 20X, driving diversity in manufacturing & services
This surge in female apprenticeship enrollment not only reflects the growing momentum towards gender equality but also signifies a paradigm shift in the roles women are undertaking
Read moreRelated Products

Programmable Controllers - Pcd-33a Series
Pro-Med Instruments (P) Ltd offers a wide range of programmable controllers - PCD-33A Series.

Gasket Graphite Powder
Arihant Packing & Gasket Company offers a wide range of gasket graphite powder.
Asahi Kasei expands 3D printing filament sales in North America
Asahi Kasei, a leading resin and compounding technology provider, has initiated the sales of 3D printing (3DP) filaments in North America through Asahi Kasei Plastics North America (APNA). The soft la Read more















