Schedule a Call Back
Yokogawa joins hand with ICQ Consultants for biopharmaceutical business in USA
 Industry News
Industry News- Mar 08,21

Related Stories
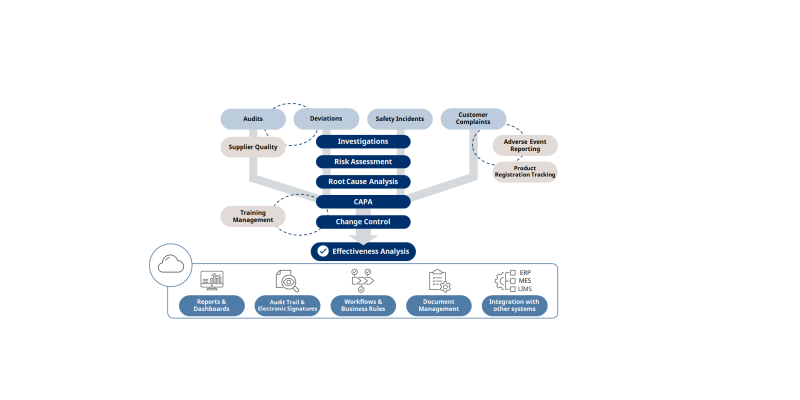
Yokogawa unveils OpreX to accelerate DX in quality assurance
The audit trail and user management methods employed by this system comply with global pharmaceutical regulations, leading to improved integrity in the handling of data for quality assurance proce..
Read more
Yokogawa unveils OpreX Subsea system to detect damage at offshore wind farms
Subsea power cables at offshore wind power generation facilities are prone to faults and damage from natural disasters and accidents, and damage to these cables can lead to significant financial los..
Read more
India urged to expand local vaccine manufacturing and research: GTRI
The think tank recommended that the Indian government investigate all adverse health events post-vaccination.
Read moreRelated Products

Programmable Controllers - Pcd-33a Series
Pro-Med Instruments (P) Ltd offers a wide range of programmable controllers - PCD-33A Series.

Gasket Graphite Powder
Arihant Packing & Gasket Company offers a wide range of gasket graphite powder.
Asahi Kasei expands 3D printing filament sales in North America
Asahi Kasei, a leading resin and compounding technology provider, has initiated the sales of 3D printing (3DP) filaments in North America through Asahi Kasei Plastics North America (APNA). The soft la Read more


















