Schedule a Call Back
Automation ensures ‘Right-First-Time’ production output: Sanjeev Dharwadkar
 Interviews
Interviews- Oct 28,24
Related Stories
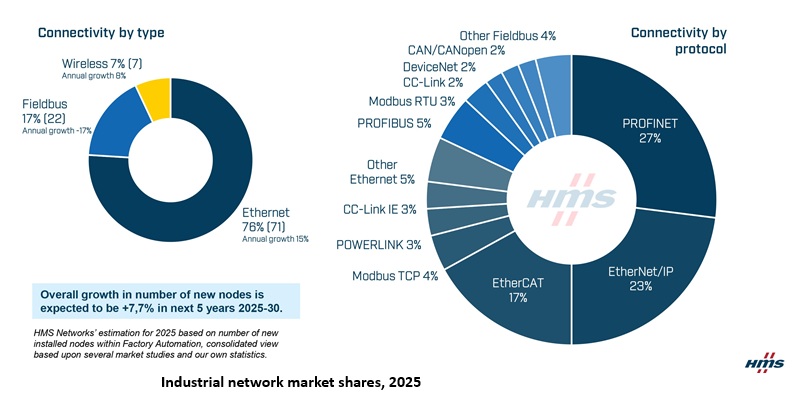
Industrial Ethernet now powers 76% of factory automation nodes: HMS Networks
HMS Networks’ 2025 report shows Industrial Ethernet now powers 76 per cent of new factory automation nodes, as fieldbus tech declines. Despite a short-term slowdown, long-term market growth is for..
Read more
DiFACTO Robotics acquires RoboFinish Division from Grind Master
RoboFinish portfolio, which includes robotic grinding, finishing, deburring, and machining technologies, presents significant synergies with DiFACTO’s existing offerings, strengthening its positio..
Read more
Phoenix Contact’s Trio Power: Reliable Power Supply for Material Handling
Phoenix Contact’s Trio Power: Reliable Power Supply for Material Handling
Read moreRelated Products

Programmable Controllers - Pcd-33a Series
Pro-Med Instruments (P) Ltd offers a wide range of programmable controllers - PCD-33A Series.

Gasket Graphite Powder
Arihant Packing & Gasket Company offers a wide range of gasket graphite powder.
Asahi Kasei expands 3D printing filament sales in North America
Asahi Kasei, a leading resin and compounding technology provider, has initiated the sales of 3D printing (3DP) filaments in North America through Asahi Kasei Plastics North America (APNA). The soft la Read more














